What Best Describes a Covalent Bond
1 removing an electron from the metal 2 adding an electron to the nonmetal and 3 allowing the metal cation and nonmetal anion to come together. Properties of Covalent Bond If sharing a single electron pair between atoms does not satisfy an atoms normal valence the atoms may share more than one electron pair between them.

Covalent Bond Definition Types And Examples
The wick is usually surrounded by wax.

. A brief treatment of covalent bonds follows. Covalent bonds have the following properties. Covalent bonds can be best described as neutral atoms coming together to share electrons.
Covalent bonding results in the formation of molecules or giant structures. A bond formed by the sharing of protons. Valence electrons are transferred from one atom to the other.
A covalent bond may also be termed a molecular bond. The atoms valence electrons combine to form a network of bonds. Which linkage best describes the covalent bond between an amino acid AA and its cognate tRNA.
Covalent bonds are magical. A bond formed by the sharing of neutrons. A covalent bond is.
Which statement correctly describes a covalent bond. Answer choices -1 1-2 2. Covalent bond in chemistry the interatomic linkage that results from the sharing of an electron pair between two atoms.
Bonds form between nonmetal atoms Diatomic molecule a molecule that consists of two atoms. Which of the following describes covalent bonds. Identify which of the following statements best describes a covalent bond.
A pair of electrons shared between two atoms where each atom has donated one electron. Covalent bonds can form between atoms of the same element. The atoms valence electrons are shared between the atoms.
Which best describes most covalent compounds. Carboxyl group of AA linked to 3 OH of tRNA carboxyl group of AA linked to 3 phosphate of tRNA amino group of AA linked to 5 OH of tRNA amino group of AA linked to 5 phosphate of tRNA none of the above. A covalent bond forms when the bonded atoms have a lower total energy than that of widely separated atoms.
Covalent bonds are formed when atoms share on or more electrons. Think of forming an ionic compound as three steps this is a simplification as with all models. Protons have a charge of.
Covalent bonds can form between atoms of different elements. Brittle A candles wick is the fabric string that holds the flame and it burns down at a constant slow pace when the candle is lit. Ionic bonds are formed between a metal and a non-metal.
After a covalent bond has stabilized an atom the atom will have a full valence electron shell. It is formed when pairs of electrons are shared by atoms. A pair of electrons shared between two atoms where one atom has donated two electrons.
Electrons are transferred between atoms. A covalent bond in chemistry is a chemical link between two atoms or ions in which the electron pairs are shared between them. The binding arises from the electrostatic attraction of their nuclei for the same electrons.
The diagrams below show the. Expert-verified answerquestionquestion mark A covalent bond is fashioned among non-metals which have comparable electronegativities. Covalent bonds can form between two nonmetal atoms.
A covalent Bond refers to such an association formed by the sharing of electron pairs among different or similar kinds. A bond formed by the sharing of electrons. A covalent bond is a shared pair of electrons.
In general what is the sign of the sum of the first two processes. Phosphorous P and chlorine Cl bond covalently to form the important industrial compound phosphorous trichloride. Covalent bonds form between two nonmetal atoms with identical or relatively close electronegativity values.
Covalent bonds are bonds formed between two non-metals. Two pairs of electrons shared between two atoms where each atom has donated. Neither atom is strong.
Which of these statements best describes a dative covalent bond. A bond formed by the gain of electrons. Bonds form to fill outer electron shells.
Bonds form because of opposite charges. Examples include fluorine F2 and hydrogen H2. Covalent bond a chemical bond in which atoms share a pair of valence electrons.
All of the atoms electrons are shared between the atoms please help. A bond formed by the loss of electrons. Atoms will covalently bond with different atoms that allows you to benefit extra.
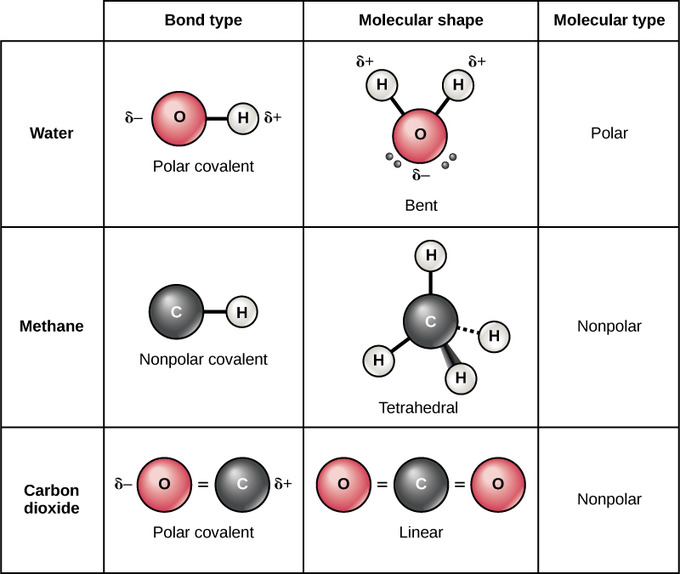
Molecules with more than two atoms are polyatomic molecules Lewis diagram.

Covalent Bond Definition Types And Examples
2 1i Covalent Bonds And Other Bonds And Interactions Biology Libretexts

Covalent Bond Definition Properties Examples Facts Britannica

No comments for "What Best Describes a Covalent Bond"
Post a Comment